Good morning, dear readers! Take a moment to savor the day as we delve into the intriguing world of pharmaceuticals. At the Pharmalot campus, amidst rising temperatures and hazy skies, there’s an air of anticipation. The birds’ melodies harmonize with a gentle breeze, creating a serene atmosphere.
Picture this – our official mascots lounging around, their rhythmic breaths blending with the tranquility of the surroundings. As we prepare our daily dose of stimulation (butter pecan today for those curious taste buds), we extend a warm invitation to you to partake in this journey with us.
Let’s dive into gripping tales from the pharmaceutical realm that promise to enlighten and captivate your imagination. Buckle up for an exploration filled with intrigue and revelations that await in the corridors of drug development.
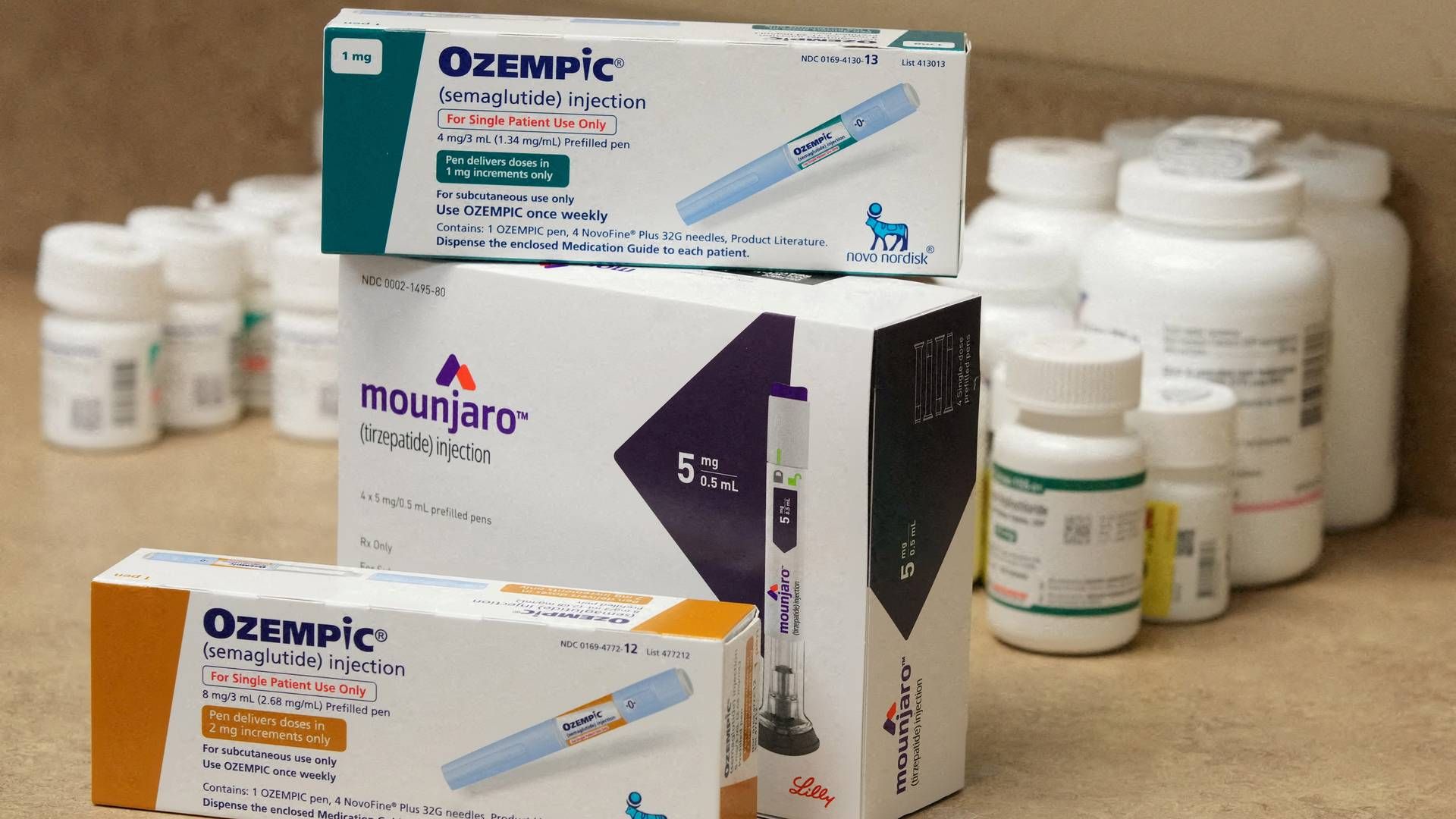
The Tale of Novo Nordisk’s Innovation
In the early 2010s, Novo Nordisk embarked on a groundbreaking venture by developing a revolutionary weekly treatment for obesity. This innovative therapy targeted three vital hormones simultaneously – GLP-1, GIP, and glucagon. Initial studies conducted on mice revealed promising results in terms of significant weight loss attributed to this novel drug.
However, despite these encouraging outcomes, Novo made a prudent decision to shelve this pioneering therapy due to concerns regarding potential side effects associated with targeting glucagon. The apprehension stemmed from possible repercussions such as elevated blood sugar levels and increased heart rate.
One cannot help but marvel at Novo’s foresight in prioritizing patient safety above all else. The company already had another promising obesity treatment named Wegovy in its pipeline – a GLP-1 drug showing immense potential. This strategic move highlighted Novo’s commitment to responsible healthcare practices while showcasing their dedication to enhancing patient outcomes.
Former employees shed light on various factors contributing to Novo’s cautious approach – from inheriting a conservative organizational culture to historically investing less in research and development compared to industry peers. Their unwavering belief in Wegovy might have inadvertently overshadowed other equally potent drug candidates waiting in the wings.
Eli Lilly’s Bold Leap
While Novo Nordisk opted for prudence, Eli Lilly seized the opportunity by swiftly advancing its own triple agonist therapy. Today, Eli Lilly stands at the forefront with its medication undergoing late-stage testing and earning accolades as one of the most promising contenders within the obesity drug landscape.
Unveiling FDA Approval Realities
Diving deeper into regulatory realms unveils startling insights surrounding U.S. Food and Drug Administration (FDA) approvals over recent years. A meticulous study by The Lever and McGraw Center for Business Journalism exposed a stark reality – majority of drugs receiving FDA approval failed to meet essential efficacy standards while posing serious health risks.
Shocking revelations highlight how nearly three-quarters of approved drugs fell short in providing substantial evidence validating their anticipated efficacy levels. More alarmingly, over half of these approvals relied on preliminary data rather than concrete proof demonstrating improved patient symptoms or enhanced functionality post-treatment.
Of particular concern were cancer drugs receiving regulatory nods without compelling evidence substantiating their efficacy claims – only three out of 123 approved cancer medications met all stringent scientific criteria set forth by the FDA guidelines.
These findings underscore critical gaps within current regulatory frameworks necessitating rigorous evaluation protocols ensuring patients receive safe and efficacious treatments devoid of undue risks or uncertainties.






Leave feedback about this