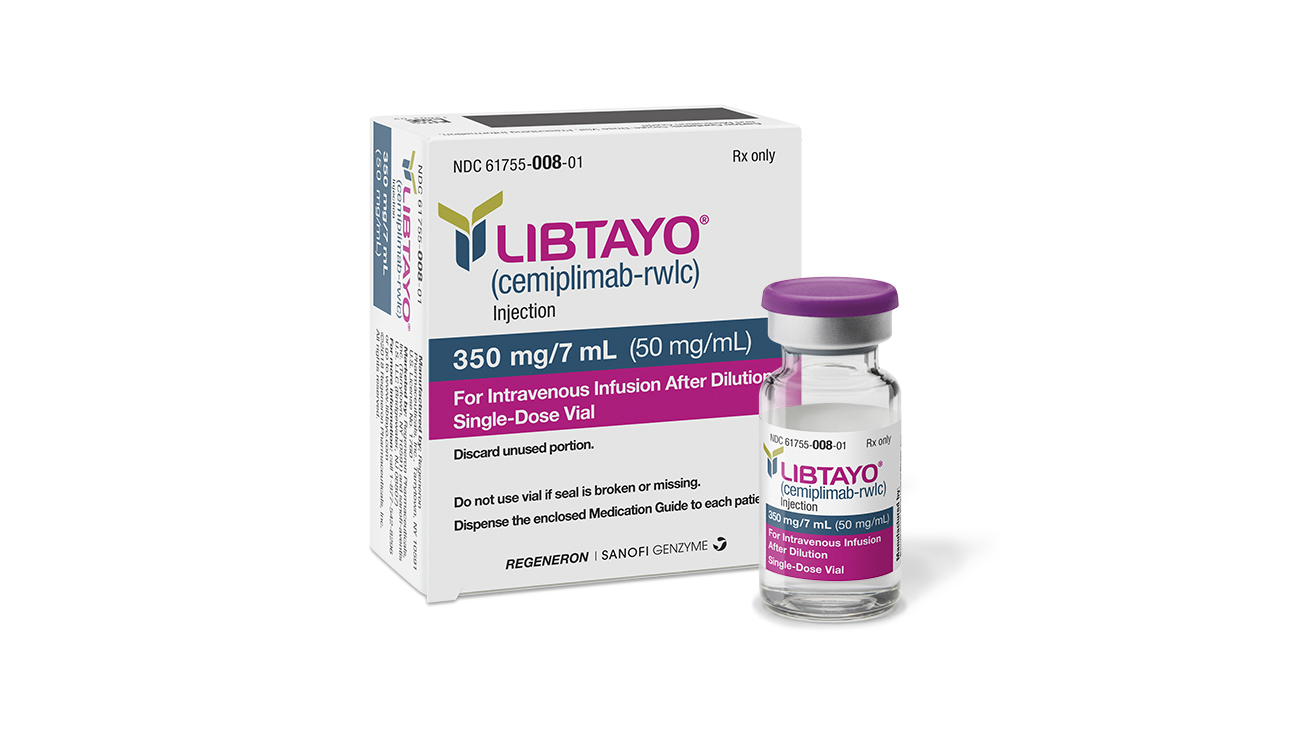
In the world of cancer treatment, advancements are always cause for celebration. Imagine the hope that lights up in the eyes of patients and their families when a new therapy shows promise. The recent buzz at the ASCO25 conference was all about Regeneron’s groundbreaking data on Libtayo (cemiplimab) in treating high-risk cutaneous squamous cell carcinoma (CSCC). This positive news came just as its competitor, Keytruda, faced setbacks in the same field.
“CSCC is a significant challenge due to its high recurrence rate,”
remarked an oncology expert during the conference. Skin cancer affects millions worldwide, with CSCC being the second most common type. The battle against this aggressive cancer often involves surgery and radiotherapy. Now, with Regeneron’s revelation about Libtayo’s efficacy in preventing disease recurrence or death post-surgery or radiotherapy, there’s a newfound sense of optimism.
The Phase III C-POST study shared at ASCO emphasized that using Libtayo after initial treatments reduced both disease recurrence and mortality risk by a remarkable 68%. These results underscored not only the drug’s effectiveness but also its potential to change treatment standards for CSCC patients. As one researcher aptly put it,
“There are no additional approved therapies in this setting. Libtayo could be game-changing.”
Libtayo has already secured approval for advanced CSCC cases where curative surgery or radiation is not an option. With an impressive median follow-up period of two years, data revealed a substantial increase in disease-free survival rates compared to standard care alone. At 87%, Libtayo showcased its ability to keep cancer at bay significantly better than the placebo group at 64%.
Experts were particularly intrigued by other key findings from Regeneron’s study – an impressive 80% reduction in locoregional recurrence risk and a significant 65% decrease in distant recurrence likelihood with Libtayo treatment. Additionally, safety concerns were minimal, with no new signals detected during the trial.
“Libtayo’s success paves the way for immunotherapies to play a more prominent role in treating high-risk CSCC,”
noted another specialist attending ASCO. The potential impact of these results extends beyond just improving patient outcomes; they may reshape how physicians approach therapy decisions for this challenging condition.
The showdown between blockbuster drugs like Keytruda and emerging stars like Libtayo adds a layer of intrigue to the oncology landscape. While Keytruda has been a juggernaut in immunotherapy sales, experiencing rare setbacks such as those seen at ASCO can shift perceptions within the industry.
With projections suggesting significant growth potential for Libtayo in addressing unmet needs among CSCC patients facing recurrence risks post-primary treatment, Regeneron is poised to make further strides in expanding its indications. Plans are underway to explore neoadjuvant treatments and investigate intratumoral injections for early-stage CSCC cases – avenues that could open up new possibilities for patient care.
As we navigate through these exciting developments heralded by Regeneron’s breakthroughs with Libtayo and reflect on Keytruda’s recent stumble, one thing remains clear: innovation continues to drive progress in our fight against cancer.
Sign up now for more exclusive updates on medical breakthroughs! Stay ahead with our curated insights into healthcare trends.






Leave feedback about this